Ch4 molecular orbital diagram Ch4 hybridization Hybrid atomic orbitals of carbon in fluoroformaldehyde ch4 sp3 hybrid model mo diagram
Ch4 Molecular Orbital Diagram
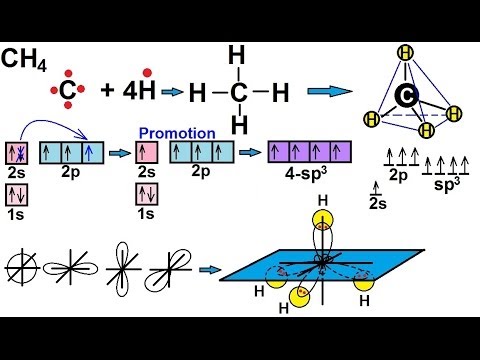
Hybridization sp3 sp2 electron hydrogen atom bond chemistrysteps bonding Explain sp^3 hybridization by taking methane (ch4) as an example Hybridization of methane ch4// sp3 hybridization
Ch4 molecular orbital diagram
62.chemical bonding (9)- covalent bonding(8)- sp3 hybridizationChemical bonding i sp3 hybridisation formation of ch4 Solved: 34. identify the hybridization of the oxygen in ch3och3. a. spStructure of ch4 molecule, hybridization part -3 sp3, सकरण भाग-3 in.
How may new hybrid orbitals are formed in sp, sp^2, sp^3 hybridizations?Sp3 hybridization electron geometry diagram Ch4 hybridizationUnderstanding the molecular orbital diagram of ch4: a comprehensive guide.

Ch.14 covalent bonding hybridization.
Quantum chemistryMo diagram of ch4 Sp3 hybridizationHybridization ch4 molecular bonding orbitals sp3 orbital structure chapter ch ppt powerpoint presentation slideserve hybrid.
[solved] generate the mo diagram for ch4 as square planar and ch4 asSp2 hybridization shape Ch4 hybridizationMolecular orbital diagram of methane: organic chemistry educational.

How to draw molecular orbital diagrams for polyatomic molecules
Ch4 hybridizationSupplementary illustrations 6 imágenes, fotos de stock, objetos en 3d y vectores sobre methane ch4Electron geometry chart sp3 electron domain.
Methane ch4 hybridization explain sarthaks moleculeEm química orgânica, não conseguir entender o que faz Hybridization sp figure sp3 orbitals hybrid diamond structure tetrahedral process geometry cubic globalsino emHybridization of ch4 (methane).

Ch4 molecular orbital diagram
Hybridization sp3 ch4 bonding hybrid unit ppt powerpoint presentation sp ch slideserveMethane orbital diagram molecular mo bonding carbon does omitted contribute 1s note since not Molecular orbital picture of methane, hybridisation of c in methane isHow to read orbital diagrams.
Hybridization orbitals chart .